Crude oil emulsion
definition
A crude-oil emulsion is a dispersion of water droplets in oil. Produced oilfield emulsions can be classified into three broad groups:
definition
A crude-oil emulsion is a dispersion of water droplets in oil. Produced oilfield emulsions can be classified into three broad groups:
- Water-in-oil (W/O) emulsions.
- Oil-in-water (O/W) emulsions.
- Multiple or complex emulsions.
Factor that affecting the stability
- Heavy polar material in crude oil
- Asphaltenes
- Resins
- Oil-soluble organic acids and base
Asphaltenes
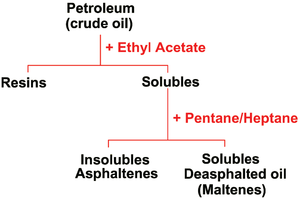
Asphaltenes are complex polyaromatic molecules. Soluble in benzene/ethyl acetate. Insoluble in low-molecular-weight alkanes. They are friable solids with no definite melting point. They are considered to consist of condensed aromatic sheets with alkyl and alicyclic side chains and heteroatoms (Nitrogen, Oxygen, Sulfur, Trace metals) scattered throughout.
Asphaltenes are complex polyaromatic molecules. Soluble in benzene/ethyl acetate. Insoluble in low-molecular-weight alkanes. They are friable solids with no definite melting point. They are considered to consist of condensed aromatic sheets with alkyl and alicyclic side chains and heteroatoms (Nitrogen, Oxygen, Sulfur, Trace metals) scattered throughout.
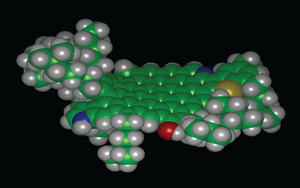
3D representation of a Venezualan asphaltene molecule (courtesy of J. Murgich and A. Mansoori).
two factors control emulsion stability: the amount of asphaltenes and the aromatic/alkane ratio in the crude oil. Emulsification tendencies reduce with increasing aromatic content of the crude oil. Asphaltenes, apart from stabilizing emulsions themselves, alter the wettability of other solids present and make them act as emulsifying agents for water-in-oil emulsions
two factors control emulsion stability: the amount of asphaltenes and the aromatic/alkane ratio in the crude oil. Emulsification tendencies reduce with increasing aromatic content of the crude oil. Asphaltenes, apart from stabilizing emulsions themselves, alter the wettability of other solids present and make them act as emulsifying agents for water-in-oil emulsions
Resins
They are complex high-molecular-weight compounds. Insoluble in ethyl acetate but soluble in n-heptane. They are hetero compounds that contain Oxygen, Nitrogen, and Sulfur atoms. It appears that the asphaltene-resin ratio in crude oil is responsible for the type of film formed (solid or mobile) and, therefore, is directly linked to the stability of the emulsion. There are two types of petroleum waxes: Paraffin and microcrystalline. Paraffin waxes are high-molecular-weight normal alkanes, and microcrystalline waxes are high-molecular-weight iso-alkanes that have melting points greater than 50°C. The physical state of the wax in the crude oil also plays an important role in emulsion stabilization. Waxes are more apt to form a stable emulsion when they are present as fine solids in the emulsion. Waxes, being oil-wet, have a tendency to stabilize water-in-oil emulsions. Crudes that have a high cloud point generally have a greater tendency to form stable and tight emulsions than crudes with low cloud points. Similarly, lower temperatures generally enhance the emulsion-forming tendencies of crude oils.
They are complex high-molecular-weight compounds. Insoluble in ethyl acetate but soluble in n-heptane. They are hetero compounds that contain Oxygen, Nitrogen, and Sulfur atoms. It appears that the asphaltene-resin ratio in crude oil is responsible for the type of film formed (solid or mobile) and, therefore, is directly linked to the stability of the emulsion. There are two types of petroleum waxes: Paraffin and microcrystalline. Paraffin waxes are high-molecular-weight normal alkanes, and microcrystalline waxes are high-molecular-weight iso-alkanes that have melting points greater than 50°C. The physical state of the wax in the crude oil also plays an important role in emulsion stabilization. Waxes are more apt to form a stable emulsion when they are present as fine solids in the emulsion. Waxes, being oil-wet, have a tendency to stabilize water-in-oil emulsions. Crudes that have a high cloud point generally have a greater tendency to form stable and tight emulsions than crudes with low cloud points. Similarly, lower temperatures generally enhance the emulsion-forming tendencies of crude oils.
2. Fine Solids
Fine Solids as Stabilizers
1. Fine solids, such as clay particles (e.g., montmorillonite) and silt, are naturally present in many crude oil reservoirs. These fine solids can become dispersed in the emulsion and serve as stabilizers through various mechanisms.
2. Adsorption at the Interface: Fine solids migrate to the oil-water interface and form a layer around the water droplets. This layer helps to stabilize the emulsion by preventing the coalescence of water droplets.
3. Steric and Electrostatic Repulsion: Fine solids can also possess surface properties that lead to steric stabilization and electrostatic repulsion. This makes it more difficult for water droplets to merge or coalesce, contributing to emulsion stability.
1. Fine solids, such as clay particles (e.g., montmorillonite) and silt, are naturally present in many crude oil reservoirs. These fine solids can become dispersed in the emulsion and serve as stabilizers through various mechanisms.
2. Adsorption at the Interface: Fine solids migrate to the oil-water interface and form a layer around the water droplets. This layer helps to stabilize the emulsion by preventing the coalescence of water droplets.
3. Steric and Electrostatic Repulsion: Fine solids can also possess surface properties that lead to steric stabilization and electrostatic repulsion. This makes it more difficult for water droplets to merge or coalesce, contributing to emulsion stability.
3. Temperature
Temperature can affect emulsion stability significantly. Temperature affects the physical properties of oil, water, interfacial films, and surfactant solubilities in the oil and water phases. These, in turn, affect the stability of the emulsion. Perhaps the most important effect of temperature is on the viscosity of emulsions because viscocity decreases with increasing temperatures.
Temperature can affect emulsion stability significantly. Temperature affects the physical properties of oil, water, interfacial films, and surfactant solubilities in the oil and water phases. These, in turn, affect the stability of the emulsion. Perhaps the most important effect of temperature is on the viscosity of emulsions because viscocity decreases with increasing temperatures.
|
4. Droplet size and droplet size distribution
"Droplet size" in an emulsion refers to the width or diameter of the individual oil droplets within the emulsion. It can be measured in units such as centimeters (cm) or micrometers (µm). The droplet size of the oil provides an understanding of how small or large the individual oil droplets are within the emulsion. The size of these oil droplets can vary depending on the conditions and processes involved in creating the emulsion, such as agitation, elevated temperatures, or the use of chemicals during the mixing process. |
"Droplet Size Distribution" in an emulsion refers to how the oil droplets are distributed across a range of sizes within the emulsion. It describes the variation in droplet sizes present in the emulsion. This distribution can include both small and large oil droplets, and it provides insights into the diversity of droplet sizes within a particular emulsion. One common example used to describe droplet size distribution is the "crude oil droplet size distribution," which illustrates the range of droplet sizes found in a study of crude oil emulsion. |
Effect of droplet distribution on rheological properties of water-in-oil emulsion in waxy crude oils
- Microstructure Observation: Microscope images were used to observe and capture the microstructure of the crude oil emulsion. The study found that as the water cut (the proportion of water in the emulsion) increased, the number of dispersed droplets also increased. Furthermore, there was a shift in the quantitative proportion of droplets, with relatively larger droplets becoming more prominent compared to smaller droplets.
- Effect of Stirring Speed: The study varied the stirring speed during emulsion preparation and observed its impact. Increasing the stirring speed led to an increase in the number of dispersed droplets, while the Sauter mean diameter of droplets decreased. This suggests that higher stirring speeds can result in finer droplet sizes in the emulsion.
- Rheological Properties: Three different waxy crude emulsions were prepared at various water cuts while maintaining a constant stirring speed. The rheological properties, particularly yield stress and thixotropy near the gel points, were measured. The results showed that as the water cut increased, the yield stress and thixotropy of the water-in-oil crude emulsions also increased. This trend was further enhanced with higher water cuts.
- Effect of Stirring Speed on Thixotropy: The study examined the effect of stirring speed on thixotropy near the gel point for emulsions with a 30% water cut. It was observed that as the stirring speed increased, the shear stress of the emulsions also increased under equivalent shear rates, leading to higher thixotropy.
- Correlation between Yield Stress and Temperature/Water Content: The study established a relationship that correlated the yield stress of the emulsion with the measured temperature and water content. This correlation had an average relative error of 9.83%, indicating a reasonably accurate prediction of yield stress based on these factors.
6. Brine Composition.
Specific ions present in the brine can also influence interfacial film behavior. The effect of brine composition on interfacial film and emulsion stability has been reported. Waters from petroleum formations generally contain many ions. Sodium and chlo- ride ions are usually present in high concentrations, while other ions are present in wide- ranging quantities. At the interface, these ions may react chemically with the hydrophilic groups to form insoluble salts. In the studies cited, an insufficient number and variety of crude oil/brine systems were tested to draw any concrete conclusions regarding the effect of brine and its composition on interfacial film and emulsion-stabilizing properties. However, the follow- ing general trends are noted.
Specific ions present in the brine can also influence interfacial film behavior. The effect of brine composition on interfacial film and emulsion stability has been reported. Waters from petroleum formations generally contain many ions. Sodium and chlo- ride ions are usually present in high concentrations, while other ions are present in wide- ranging quantities. At the interface, these ions may react chemically with the hydrophilic groups to form insoluble salts. In the studies cited, an insufficient number and variety of crude oil/brine systems were tested to draw any concrete conclusions regarding the effect of brine and its composition on interfacial film and emulsion-stabilizing properties. However, the follow- ing general trends are noted.
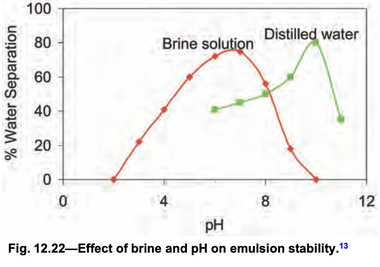
• Brine composition (alkalinity in particular because of a buffering effect) is intimately tied to the pH in determining the stabilizing properties of the interfacial films.
• Brines with high Ca++ ions and a high Ca++/Mg++ ratio form nonrelaxing, rigid films around the water droplets, resulting in stable emulsions.
• Higher concentration of divalent ions and high pH result in reduced emulsion stability. Many species of polar molecules are present at the interface, and each species responds differ- ently. Synergistic effects may occur when several different cations are present at the same time.
• Brines with high Ca++ ions and a high Ca++/Mg++ ratio form nonrelaxing, rigid films around the water droplets, resulting in stable emulsions.
• Higher concentration of divalent ions and high pH result in reduced emulsion stability. Many species of polar molecules are present at the interface, and each species responds differ- ently. Synergistic effects may occur when several different cations are present at the same time.
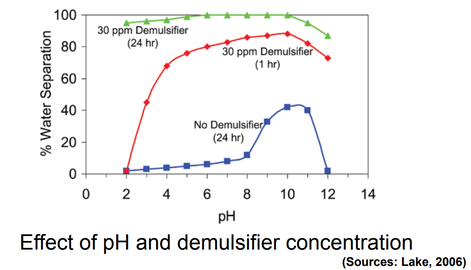
7. pH of the brine
The pH of water has a strong influence on emulsion stability. The stabilizing, rigid emulsion film contains organic acids and bases. Adding inorganic acids and bases strongly influences their ionization in the interfacial films and changes the physical properties of the films. The pH of water affects the rigidity of the interfacial films. The film becomes progressively weaker as the pH is increased.
References.
- https://petrowiki.spe.org/Stability_of_oil_emulsions#Heavy_polar_fraction_in_crude_oil
- https://onepetro.org/PO/article/20/01/5/112495/Crude-Oil-Emulsions-A-State-Of-The-Art-Review
- https://www.researchgate.net/publication/286955965_Effect_of_droplet_distribution_on_rheological_properties_of_water-in-oil_emulsion_in_waxy_crude_oils
- Petroleum Engineering Handbook ,Larry W. Lake, Editor-in-Chief I
- https://petrowiki.spe.org/PEH:Crude_Oil_Emulsions#Stability_of_Emulsions